Can water, in its solid crystalline form, truly be considered a mineral? This proposition may seem unconventional. The common perception of minerals often conjures images of glistening quartz, metallic pyrite, or vibrant tourmaline. Ice, on the other hand, is something we encounter daily – in our drinks, during winter storms, and perhaps taken for granted.
However, the geological sciences, with their precise definitions, offer a surprising perspective. Prepare to delve into the fascinating parallels between commonplace ice and the diverse world of mineralogy. The similarities are more profound than you might initially presume.
Defining the Mineral Kingdom: A Rigorous Framework
To understand ice’s potential mineral status, it is essential to clarify what constitutes a mineral in the first place. Mineralogists adhere to a stringent set of criteria. A substance must meet all these qualifications to earn the title of “mineral”.
These crucial criteria are:
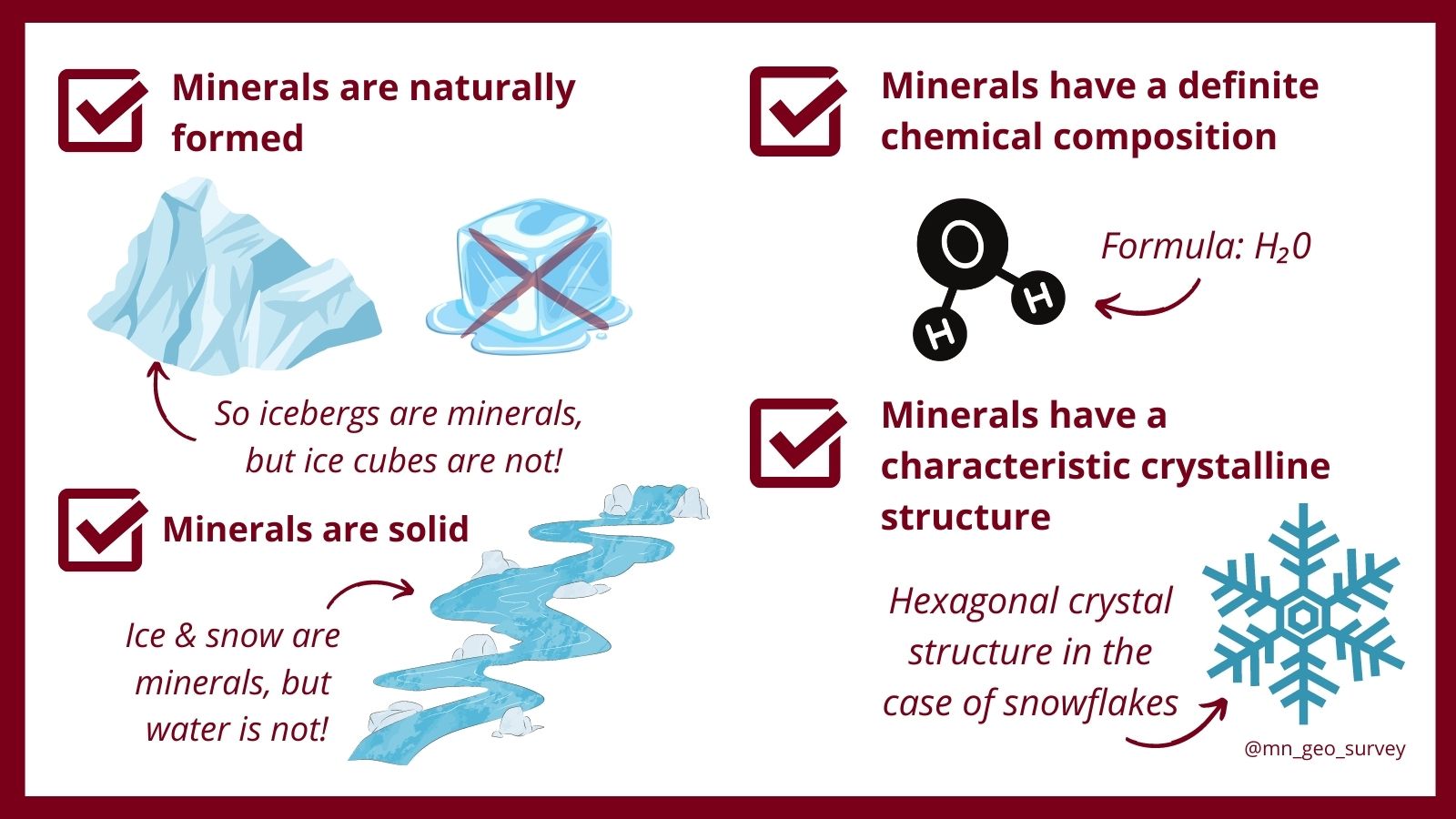
- Naturally Occurring: A mineral must be formed by natural geological processes, without human intervention. Synthetic diamonds, grown in a laboratory, may possess identical chemical and physical properties to natural diamonds. However, they do not qualify as minerals. Nature must be the artisan.
- Solid: Minerals exist in a solid state at standard temperature and pressure. This excludes liquids like water and gases like atmospheric nitrogen, despite their specific chemical compositions.
- Definite Chemical Composition: A mineral must have a relatively fixed chemical formula. This doesn’t necessarily mean the composition is absolutely immutable. Some minerals exhibit isomorphic substitution, where certain elements can replace others within the crystal structure without drastically altering it. Olivine, (Mg,Fe)2SiO4, is an excellent illustration of this phenomenon. The ratio of magnesium (Mg) to iron (Fe) can vary, yet it remains within the olivine compositional range.
- Ordered Atomic Arrangement: This is perhaps the most crucial criterion. Atoms within a mineral must be arranged in a highly ordered, repeating three-dimensional pattern. This arrangement defines the mineral’s crystal structure and dictates many of its physical properties, such as cleavage, hardness, and optical characteristics. Amorphous substances, like volcanic glass (obsidian), lack this ordered structure and are thus classified as mineraloids, not minerals.
- Inorganic: Minerals are not formed by organic processes. Substances produced by living organisms, such as pearls or coal, are excluded from mineral classification. There are some exceptions and ongoing debates, particularly concerning biogenic minerals, but the general rule holds true.
Ice: An Unexpected Mineral Contender
Now, let us evaluate ice against these established criteria. Does it fulfill the necessary qualifications?
- Naturally Occurring: Ice forms naturally in countless environments, from glaciers and polar ice caps to frozen lakes and snowflakes. No human intervention is needed for its genesis.
- Solid: Under freezing temperatures, water transitions into its solid form, ice. Therefore, it meets the solidity requirement.
- Definite Chemical Composition: Ice possesses a simple and well-defined chemical formula: H2O. Its composition is consistent and unwavering.
- Ordered Atomic Arrangement: This is where the magic truly happens. Water molecules in ice are arranged in a specific, repeating hexagonal crystal lattice. This crystalline structure imparts ice with its characteristic properties, such as its relatively low density compared to liquid water and its ability to refract light, creating stunning optical phenomena.
- Inorganic: Water, and consequently ice, is an inorganic substance. It is not a product of biological processes.
Variations in Ice: Polymorphism and Phase Transitions
The story of ice doesn’t end with simple hexagonal ice (Ice Ih), the familiar form we encounter in everyday life. Under varying pressure and temperature conditions, water can crystallize into at least 19 different crystalline forms, known as ice polymorphs (Ice II, Ice III, Ice IV, Ice V, Ice VI, Ice VII, Ice VIII, Ice IX, Ice X, Ice XI, Ice XII, Ice XIII, Ice XIV, Ice XV, Ice XVI, Ice XVII, Ice XVIII, Ice XIX). These polymorphs exhibit diverse crystal structures and physical properties. These exotic ices typically occur under extreme conditions, such as those found deep within planetary interiors. The existence of these various forms of ice further solidifies its standing as a mineral, showcasing its capacity to adapt and transform under different environmental pressures.
These phase transitions, where ice transforms from one crystalline structure to another, are analogous to the polymorphism observed in other minerals, like carbon (which can exist as graphite or diamond).
The Ubiquity and Importance of Ice as a Mineral
Accepting ice as a mineral might seem like a mere technicality, but it holds significant implications. Recognizing ice within the mineralogical framework highlights its crucial role in geological processes. Glaciers, vast rivers of ice, sculpt landscapes through erosion and deposition. Ice influences weathering patterns and plays a crucial role in the Earth’s climate system.
Moreover, understanding the properties of ice is paramount in fields like cryology (the study of ice and its properties) and planetary science, where icy bodies, such as Europa and Enceladus, hold tantalizing clues about the potential for extraterrestrial life. By acknowledging ice’s rightful place among minerals, we gain a deeper appreciation for its multifaceted nature and its profound impact on our planet and beyond.
In conclusion, while it may challenge our conventional understanding, ice unequivocally satisfies all the criteria to be classified as a mineral. Its natural occurrence, solid state, definite chemical composition, ordered atomic arrangement, and inorganic nature firmly establish its mineralogical credentials. It serves as a salient reminder that the world of minerals extends beyond the glittering gems and rare crystals, encompassing even the seemingly mundane, yet incredibly versatile, substance we know as ice.








Leave a Comment