The acronym “EtOH” frequently surfaces in scientific, medical, and industrial contexts. Its concise form often obscures its full meaning for the uninitiated. Understanding EtOH’s genesis and application requires a delve into the nomenclature of organic chemistry and its everyday manifestations.
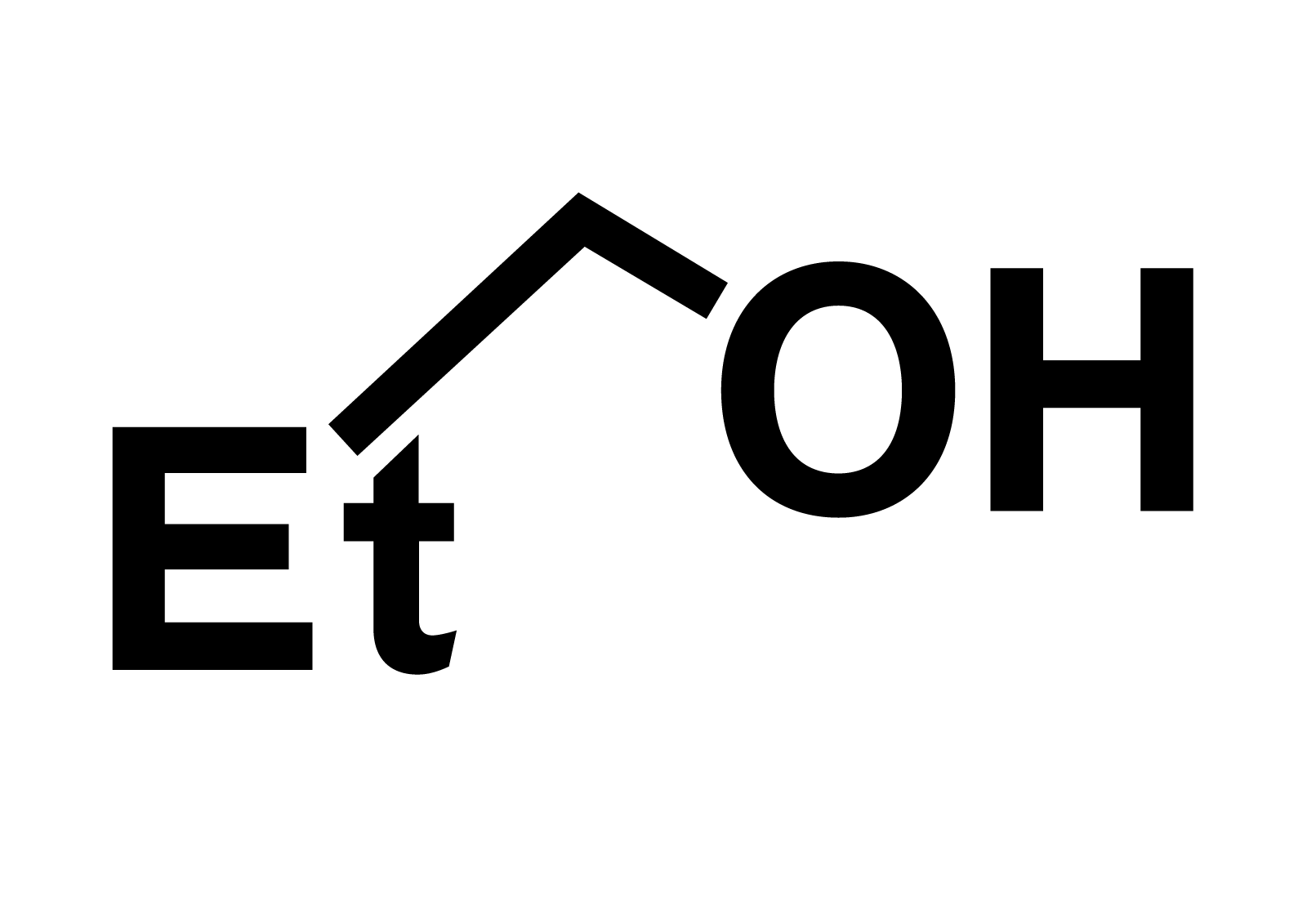
EtOH is a shorthand representation of ethyl alcohol, more formally known as ethanol. Let’s dissect this appellation. “Ethyl” denotes a chemical moiety—specifically, the two-carbon alkyl group (C2H5). This fragment constitutes a fundamental building block in organic chemistry. “Alcohol,” in its broadest sense, designates any organic compound wherein a hydroxyl group (-OH) is bonded to a saturated carbon atom.
Ethanol, therefore, signifies a molecule comprised of an ethyl group covalently linked to a hydroxyl group. This seemingly simple molecular structure belies its profound impact on human society.
A Ubiquitous Solvent and Reactant: The Essence of EtOH’s Utility
Ethanol’s versatility stems from its unique amphiphilic nature. The ethyl group imparts a hydrophobic (water-repelling) characteristic, while the hydroxyl group confers hydrophilicity (water-attracting). This dual affinity renders ethanol an excellent solvent for a wide range of substances, both polar and nonpolar. Imagine it as a molecular bridge, facilitating the dissolution of disparate entities.
The applications of ethanol as a solvent are legion:
- Pharmaceuticals: Ethanol serves as a vehicle for delivering medications, dissolving active ingredients, and promoting their absorption. It’s a silent partner, ensuring therapeutic efficacy.
- Cosmetics: From perfumes to lotions, ethanol helps to homogenize formulations, imparting a pleasant feel and aiding in the dispersion of fragrances. Think of it as the invisible hand blending disparate elements into a cohesive whole.
- Industrial Processes: Ethanol facilitates the synthesis of a multitude of chemicals, acting as a reaction medium and a cleaning agent. It’s a workhorse, diligently enabling complex transformations.
Beyond its solvent properties, ethanol participates directly in a vast array of chemical reactions. Its hydroxyl group is highly reactive, allowing for the formation of esters, ethers, and other valuable compounds. This reactivity makes ethanol a key intermediate in the production of plastics, detergents, and numerous other industrial products.
Ethanol’s Entanglement with Societal Fabric: A History Steeped in Culture
Ethanol’s most widely recognized role lies in the realm of alcoholic beverages. Fermentation, the process of converting sugars into ethanol and carbon dioxide by microorganisms, has been practiced for millennia. From ancient Sumerian beer to modern-day wines and spirits, ethanol has been a central element of human culture.
The effects of ethanol on the human central nervous system are complex and multifaceted. At low concentrations, it can induce feelings of relaxation and euphoria. However, as the concentration increases, ethanol impairs cognitive function, motor coordination, and judgment. The fine line between pleasure and peril is a testament to ethanol’s potent physiological effects.
The regulation and taxation of alcoholic beverages have been a source of contention throughout history. Governments have grappled with balancing the economic benefits of alcohol production with the societal costs of alcohol abuse. The ongoing debate highlights the enduring tension between individual liberty and public health.
Fueling the Future: Ethanol as a Renewable Energy Source
In recent decades, ethanol has emerged as a promising alternative fuel. Bioethanol, produced from renewable sources such as corn, sugarcane, and cellulosic biomass, offers a potentially sustainable alternative to fossil fuels. The premise is simple: plants capture atmospheric carbon dioxide during photosynthesis, and the subsequent fermentation process releases that carbon back into the atmosphere when the ethanol is burned. This closed-loop system, in theory, reduces the net carbon footprint of transportation.
The reality, however, is more nuanced. The energy required to grow, harvest, and process biofuel feedstocks can offset some of the environmental benefits. The impact on land use, water resources, and food prices also requires careful consideration. The transition to a bioethanol-based economy presents both opportunities and challenges.
Despite these complexities, ethanol remains a viable component of a diversified energy portfolio. Ongoing research is focused on improving the efficiency of bioethanol production, reducing its environmental impact, and expanding the range of feedstocks that can be utilized.
A Deeper Dive: Denatured Alcohol and Industrial Applications
Pure ethanol is relatively expensive to produce and is heavily taxed in many jurisdictions due to its use in alcoholic beverages. To circumvent these regulations, industrial ethanol is often “denatured.” This process involves adding substances that render the ethanol unfit for human consumption without chemically altering its properties for industrial use. Common denaturants include methanol, isopropyl alcohol, and various bitterants.
Denatured alcohol finds widespread application in a diverse range of industrial processes:
- Cleaning and Disinfection: Ethanol’s antimicrobial properties make it an effective disinfectant for surfaces and equipment. It disrupts the cell membranes of bacteria and viruses, rendering them inactive.
- Laboratory Reagent: Ethanol is a staple in research laboratories, serving as a solvent, a preservative, and a reagent in countless experiments. Its purity and consistency are crucial for obtaining reliable results.
- Automotive Industry: Ethanol is used as a fuel additive to increase octane rating, reduce emissions, and prevent fuel line freezing. It contributes to the performance and efficiency of internal combustion engines.
The versatility of denatured alcohol underscores ethanol’s indispensable role in modern industry and technology.
In summation, EtOH, or ethyl alcohol, is far more than a simple chemical formula. It is a linchpin of countless industries, a cornerstone of human culture, and a potential pathway to a more sustainable future. From its role as a versatile solvent to its potential as a renewable fuel, ethanol’s impact is pervasive and enduring. Understanding the significance of “EtOH” requires appreciating the intricate interplay between chemistry, society, and the environment. Its unassuming abbreviation belies a profound and complex story.








Leave a Comment